Case Studies
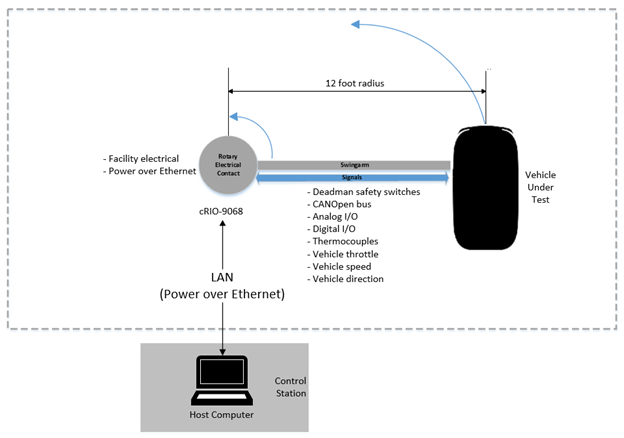
Learn how we used LabVIEW, CompactRIO and FPGA to support the development of a reliable machine control system for dynamic vehicle life testing.
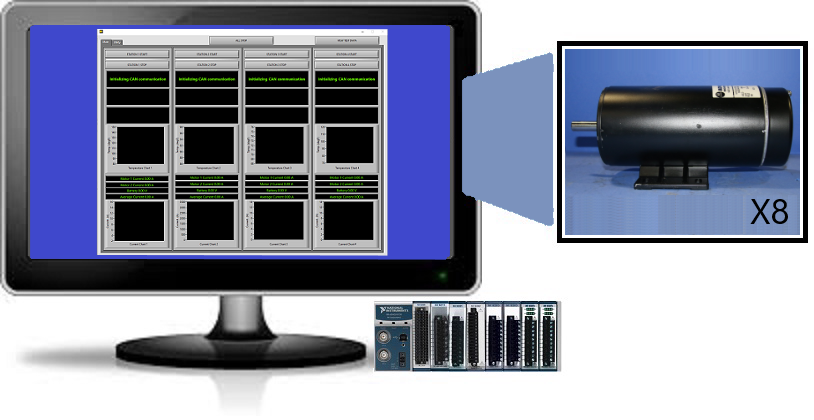
Learn how we used LabVIEW, CompactDAQ and third-party hardware to support the development of a reliable and robust design selection test system.

Learn how we used LabVIEW to support research, data collection and analysis in support of pavement design, construction and maintenance.
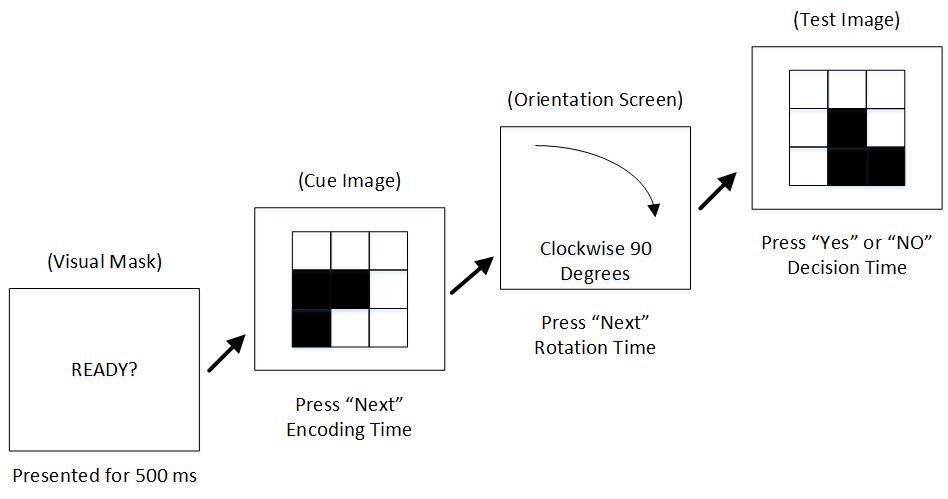
Learn how we used LabVIEW to develop and deploy a flexible, complete and easy-to-use solution for an academic research study.
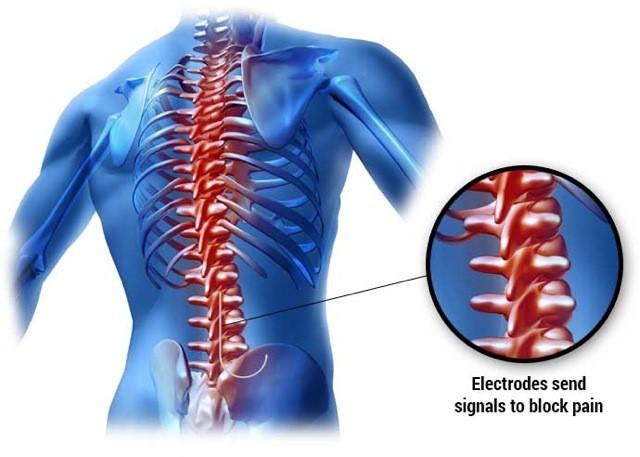
Learn how we used the advanced capabilities of LabVIEW and TestStand to develop and deploy several key components of an automated medical device test system.

Learn how we used LabVIEW and CompactDAQ to develop a custom user interface, data acquisition, data analysis, and data presentation application for an automotive starter motor test system.